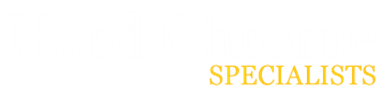If you work with equipment with metal components, you know industrial plating is crucial for a wide range of metal machinery and parts. Plating is a process of applying a layer of metal to a base component or material, primarily for added protection. Plating increases the performance of the equipment, reduces direct contact with corrosive materials, decreases friction and increases conductivity.
While understanding the importance of industrial plating may be cut and dried, choosing the right kind of plating is more complicated. The plated component's intended use will influence your decision because of the different attributes that various usages and environments require. Let’s compare two common types of metal plating — hard chrome plating and electroless nickel plating. The nickel plating vs. chrome plating debate has been a hot topic in industrial communities, and we're going to highlight the advantages and disadvantages of each plating method.
What Are the Key Differences Between Hard Chrome and Electroless Nickel Plating?
We'll assess hard chrome and electroless nickel platings in more detail later. First, let's consider some fundamental differences between the two plating methods:
- Electroless nickel plating is more resistant to corrosion.
- Hard chrome plating is generally tougher and more durable.
- Electroless nickel plating is best suited for hard-to-reach areas.
- Hard chrome offers a shiny, smooth exterior in contrast with nickel's glossier finish with a yellow hue.
What Is Hard Chrome Plating?
Chrome plating is the process of applying a layer of chromium to a metal object. Hard chrome, often called industrial chrome, differs from decorative chrome in that it is primarily functional rather than aesthetic. Decorative chrome’s fundamental purpose is to enhance the visual appeal of an object by applying a thin chrome coating. Hard chrome plating is more durable and has a broader range of applications. It's also conducive for use in many environments.
The first step in the industrial chrome plating process is typically degreasing and cleaning the surface to prepare it for the chromium application. This surface is known as the substrate. This component may also need other types of pretreatment depending on its makeup. The plating professional will then lower it into an electrochemical bath until they achieve the desired thickness.
Hard Chrome Plating Process
How Is Hard Chrome Plating Applied?
Hard chrome plating involves an electrolytic process that adds a layer of chromium to a metal component inside an electrolytic bath.
The chrome plating process typically includes these stages:
- Remove any heavy soiling by degreasing the metal component.
- Clean the substrate thoroughly to eliminate all residual dirt and surface impurities.
- Perform the appropriate pretreatments. Depending on the substrate, chemicals to improve adhesion may be necessary.
- Submerge the component into a chrome plating vat for chrome dipping, then leave it to warm to the correct temperature.
- Apply the electric current for the required time, so chromium deposits on the component surface to the desired thickness.
The chrome dipping and electrolytic process apply hard chrome plating evenly to most metal components, regardless of the shape, size or surface texture. The primary difference between hard chrome and decorative chrome plating is its final thickness. Hard chrome finishes are significantly thicker to improve their strength, wear resistance and corrosion resistance for industrial applications.
What Is Hard Chrome Plating Used For?
At the end of the hard chrome plating process, you have a smooth, functional, durable coating. Its attributes make it ideal for many different engineering applications.
The automotive industry frequently uses industrial hard chrome plating for parts that need to move while resisting wear, such as pistons and shock absorbers. In the aerospace field, landing gear components have hard chrome plating.

Often, machine tools that encounter rough use also have a hard chrome plating. These items might include drills, extrusion screws, taps and dies.
In the manufacturing sector, chrome plating shows up on gears and plastic molds, where it provides improved anti-stick and release capabilities.
Hard chrome can also restore old and worn parts or fix imperfections caused by mistakes during the manufacturing process.
What Is Electroless Nickel Plating?
The electroless nickel plating process uses a nickel-phosphorous alloy to coat a substrate, which protects it and improves its functionality. As the name suggests, electroless nickel plating doesn’t require an electric current as hard chrome plating usually does. Instead, after the substrate is clean, pretreated and activated, the nickel plating technique uses an autocatalytic chemical reaction to deposit the coating.
Hypophosphite is a commonly used agent during the electroless nickel plating process to reduce the nickel ions into a uniform coating. However, this reaction also causes varying amounts of phosphorous to persist in the plating. Electroless nickel plating can have low, medium or high levels of phosphorous. Low-phosphorous plating has approximately 2% to 5% phosphorous content. Medium has about 6% to 9%, and high has around 10% to 13%. The most typical kind is medium plating with around 8% phosphorus.
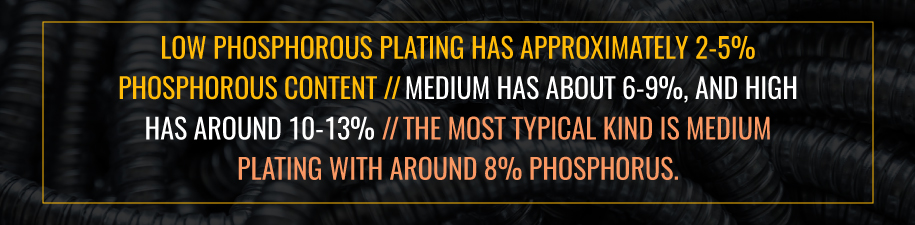
The amount of phosphorous in the plating can impact its attributes. Low-phosphorus plating provides the hardest coating, while medium plating is softer but plates the fastest. High-phosphorus plating is the softest but is the best for corrosion protection. Baking all coatings can increase hardness, but this will reduce corrosion protection.
Electroless Nickel Plating Process
How Is Electroless Nickel Plating Applied?
Electroless nickel plating uses chemical reduction to deposit a nickel-alloy coating onto components. This process does not require the electric current used in electrolytic processes, nor does it need constant filtration to prevent surface impurities.
The application process of electroless nickel plating typically includes these stages:
- Clean the substrate thoroughly to free the surface from dirt, oils and soaps formed by alkaline cleaners. Effective cleaning of the substrate is necessary to ensure proper adhesion of the nickel plating.
- Perform the appropriate pretreatments, which vary depending on the final product's intended use and the substrate itself. Careful surface preparation provides better adhesion to carbon steel.
- Place the component into a plating bath consisting of positively charged nickel-phosphorus. An autocatalytic chemical reaction will automatically draw the dissolved nickel ions onto the substrate.
- Use a chemical reducing agent, such as sodium hypophosphite, to reduce the positively charged nickel ions into a uniform layer of metallic nickel coating.
What Is Electroless Nickel Plating Used For?

Electroless nickel plating plays a role in a range of sectors, including the automotive, electronics and oil industries.
Car manufacturers use it to protect parts, such as pistons, fuel injectors and cylinders, from wear and corrosion. The aerospace industry uses it for similar reasons on valves, engine shafts and other components.
The oil and gas industry uses electroless nickel plating for equipment that will face harsh conditions underground or underwater. It’s a critical part of various pumps and pipe fittings.
It’s also common to find nickel coating in electronics such as hard drives and printed circuit boards. Like hard chrome plating, using it in molds can improve the release and anti-stick capabilities and restore worn or damaged parts. It’s also a common plating used on packaging and handling machinery.
Substrate Surfaces
Is Hard Chrome or Electroless Nickel Better for Coating Uneven Objects?
Chrome and electroless nickel plating both work well for various substrate materials. Because the coating deposits onto all surfaces of the component uniformly in the plating vat, you can use both metals for irregularly shaped objects, including those with holes and recesses.
Though both options can cover uneven objects, electroless nickel will more quickly create a uniform coating. You may need to polish chrome away in some places to create a more even covering. Electroless nickel plating also tends to result in a layer with a more constant thickness, meaning you often end up needing less nickel than you would chromium to plate an irregular object.
You can use versatile chrome on many different kinds of metals, including stainless steel, copper and brass, as well as plastic. You can also use electroless nickel on various metals and plastics.
Hardness Comparison
How Hard Is Hard Chrome Plating?
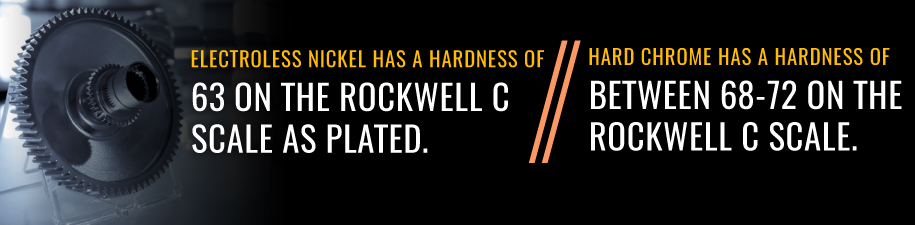
As its name implies, hard chrome is durable. This property helps it withstand the wear of industrial use, including severe mechanical contact. As plated, hard chrome Rockwell hardness is between 68 and 72.
How Hard Is Electroless Nickel Plating?
Electroless nickel can also protect components from wear and tear that occurs over time, which helps parts last longer and saves companies money on maintenance and replacement costs. Electroless nickel has a hardness of 63 on the Rockwell C scale as plated.
If wear resistance is your priority, you may choose to go with hard chrome. However, both options can protect your equipment and parts from wear.
Duration Comparison
Is Hard Chrome or Electroless Nickel Plating More Durable?
Hard chrome plating and electroless nickel plating tend to last a long time, even with consistent mechanical contact and other uses that might cause wear. Not only will plating protect the substrate, but using one of these long-lasting solutions will also save you money by cutting down on the frequency with which you need to plate your parts.
The electroless process gives the nickel in this plating application more durability. Nickel applied with a conventional electrolytic method would tarnish over a relatively short period. However, electroless nickel lasts for a much longer time without degrading, making it a more economical choice.
Corrosion Resistance
Is Hard Chrome or Electroless Nickel Plating More Resistant to Corrosion?
Along with wear resistance, one of metal plating's foremost benefits is corrosion resistance. Both hard chrome and electroless nickel can resist corrosion and protect the material from the degradation that would occur from coming into contact with its environment.

So does electroless nickel plating rust? Though both plating materials offer suitable corrosion resistance, studies show that electroless nickel is superior in this area, especially in blind holes. These holes are more challenging to plate and tend to be more susceptible to corrosion. Electroless nickel plating creates a more even coat in these hard-to-reach places, making it more effective at preventing corrosion in these areas.
Hard chrome plating does rust. Hard chrome contains tiny cracks that make it susceptible to corrosion over time. However, it has impressive corrosion resistance to several chemically corrosive sources such as sodium chloride, citric and nitric acids and copper sulfate. Moreover, the temperature affects hard chrome's resistance to rust. While both are good choices, you may want to go with electroless nickel if corrosion resistance is your priority.
Coefficient of Friction
Does Hard Chrome or Electroless Nickel Plating Have a Lower Coefficient of Friction?
The coefficient of friction refers to how easily one material can slide past another, as calculated by dividing the force needed to move the object along the other’s surface by the force that pushes them together. A low coefficient of friction means an object will slide easily, while a high one means the opposite. The coefficient depends on many factors, including the substrate, the thickness of the plating and environmental factors such as temperature.
Though calculating this number can be challenging, thanks to several tests in which these factors remained constant, we know that both hard chrome and electroless nickel have a lower static coefficient of friction than many other metals. However, hard chrome has a slightly lower coefficient than electroless nickel. It is also typically better at retaining oil, which makes movement easier. These qualities make hard chrome plating a popular choice for components that encounter a lot of friction, like pistons and hydraulic cylinders.
Electroless nickel against electroless nickel has a coefficient of 0.45. Chromium's static friction coefficient against chromium is 0.41. This means that hard chrome will likely slide better than electroless nickel, but the difference is not enough to be the deciding factor when choosing between the two materials.
Conductivity
Is Hard Chrome or Electroless Nickel Plating More Conductive?
When the ability to conduct electrical current is one of a metal's most desirable attributes, people typically go for copper. Because copper conducts electricity so readily, the International Electrotechnical Commission created a standard for commercially pure annealed copper’s conductivity, known as the International Annealed Copper Standard. To describe a metal's conductivity, you can express it as a percentage of the IACS.

On this scale, copper is 100%. Chromium is 13%, so it has 13% of copper's conductivity. For comparison, gold is around 73%, and pure silver is around 105%. Electroless nickel plating electrical conductivity is 2.2%. People don't typically use hard chrome and electroless nickel plating for their electrical conductivity attributes. They are conductive, but not as much as some other metals.
Price
Is Hard Chrome or Electroless Nickel More Expensive?
Hard chrome plating is generally more expensive than electroless nickel plating. One of the key determining factors is that hard chrome plating is in high demand because of its appearance. Also, hard chrome plating uses an electrolytic process, requiring electricity at all levels. However, the overall cost of the hard chrome might be beneficial in terms of durability over long periods. Hard chrome wears less and better suits high-friction conditions.
On the other hand, electroless nickel plating is more resistant to corrosion, meaning less maintenance and lower costs. But the difference is not significant, and both hard chrome and electroless nickel require occasional maintenance. Electroless nickel's price makes it more attractive for industry settings.
Appearance
Though appearance is not usually the primary concern in industrial uses, it’s an added benefit of choosing either hard chrome plating or electroless nickel plating. Chrome provides a shiny, smooth and modern look. Its style is part of what made it so popular.
Nickel is another classic look you can see in most home fixtures. Unlike more conventional nickel, electroless nickel plating's yellowish hue fades over time. Additionally, electroless plating provides a glossier finish than the electrolytic alternative.
Common Hard Chrome and Electroless Nickel Plating Problems and Solutions
The plating process is vital in protecting the underlying metal. However, it's common to encounter problems before or after. Here are the most common problems for hard chrome and electroless nickel plating:
Common Hard Chrome Plating Problems and Solutions
- Blistering: This is the formation of bubbles within or under a plated surface. Blistering leads to adhesion failure and damages the metal surface. To avoid this, clean the base surface thoroughly and ensure it's free from dirt before plating.
- Burnt plating deposits: Burned deposits occur around the edges and areas with a current density greater than the average current density. This causes the affected areas to burn. The precautionary measure involves controlling the current density.
- Cleavage points: These are commonly formed during die-casting when the plating splits or fractures across the crystallographic plane. This causes problems with structural rigidity and reduces the metal's toughness. The solution is to control the heating when die casting.
Common Electroless Nickel Problems and Solutions
- Metallic contamination: Metals like lead and cadmium can cause nickel plating to destabilize. The reaction leads to decomposition. To prevent this, plate on plastics and rinse the plating equipment before use, or avoid using it for materials that use precious metal catalysts entirely.
- Organic contamination: This is the most common cause of deposit pitting. Prevent organic contamination by replacing the contaminated bath and having good chemical control over subsequent baths.
- Dull deposits: Several factors cause dull deposits, including poor treatment and contaminated baths. Also, low pH and nickel activity in the bath may produce more nodular deposits. To prevent dull deposits, maintain the bath chemistry, temperature and pH, and note any pretreatment.
What to Consider When Choosing Between Hard Chrome and Electroless Nickel Plating
Choosing between hard chrome plating and electroless nickel plating can be a tricky decision. Both are useful, but the right option for you depends on how you plan to use the plated component. Here’s what you should consider when weighing your options.
- Wear: Will frequent use of the component and the other objects it encounters make it more likely to wear down over time?
- Environment: What kind of conditions will you use the component in? Consider whether you will expose it to moisture, excessive heat or cold and other environmental factors.
- Motion: Is the component a moving part that needs to run smoothly without wearing down? A part that gets stuck can slow down or halt processes and damage equipment.
- Shape of the substrate: Is the shape of the plated item relatively smooth and uniform, or is it irregular? Check for holes, indentations, abnormally shaped parts and rough surfaces.

Why Choose Hard Chrome Plating?
Once you’ve analyzed your needs for your plated component, you can determine whether hard chrome plating is the right choice for you. Hard chrome plating has the following attributes and benefits:
- Superior hardness: If you need your plating to be exceptionally hard and durable, go with hard chrome. For components that face the potential for a lot of wear, hard chrome is the best choice for a component that will function well even in harsh conditions and last longer. It also adheres well to several metal bases, making it resistant to delamination and flaking.
- Lower coefficient of friction: If the component you are plating is a moving part, opt for hard chrome. It has a low coefficient of friction, which means it’s easier for things to move along it. It also retains oil well, which improves performance.
- Conductivity: If you’re looking for the more conductive of the two, the winner is hard chrome. While it can conduct electricity, it is, of course, not nearly as conductive as metals like copper and gold.
- Wide application: Hard chrome plating applies to various metals, including stainless steel, brass and copper. This makes it suitable for users with several metallic products.
- Low-temperature application: If you often operate in cold regions, hard chrome plating is likely the best fit. The plating reacts well in low temperatures, which means better protection of the substrate metal.
Why Choose Electroless Nickel Plating?

Hard chrome is a versatile and highly functional plating material. However, in some situations, electroless nickel would be the better choice. Here’s when you should go for electroless nickel.
- Exceptional corrosion resistance: If corrosion is a significant concern, electroless nickel should be your go-to plating material. The plating's uniformity and smoothness leave no room for moisture and other elements to get through, protecting your component, helping it work better and last longer.
- Uniformity: Electroless nickel’s uniformity enhances its corrosion resistance and makes it perfect for coating irregularly shaped items. If the component has a lot of rough surfaces, holes and other hard-to-cover areas, reach for this material to ensure every inch of your equipment gets protection.
- Wide application: Electroless nickel plating applies to various base materials, including steel, stainless steel, brass and copper, whether conductive or non-conductive. This gives users a myriad of options.
- Affordability: If price is your primary consideration, electroless nickel plating is likely the best choice. The process requires no electricity, decreasing the cost. Moreover, the plating is cost-friendly due to less wasted nickel.
- Customizable finish: Electroless nickel plating offers many customizable finishes, making it attractive for different uses. You can choose from matte to bright.
Chrome vs. Nickel Plating
You might often find yourself in situations where you want to combine hard chrome's capabilities with electroless nickel.
The good news is that both materials are excellent in many of the same areas, even if one may have a slight edge. They’re resistant to wear and corrosion, support motion, conduct electricity and are ideal for use on irregularly shaped objects. Either way, you get a high-quality plating material. For this reason, you might sometimes see components with plating that combines chrome and electroless nickel.
At Hard Chrome Specialists, we’ve been helping customers for over 20 years with projects of all sizes. We pride ourselves on our friendly, honest service, quick turnaround and high-quality results. For more information about what we do or for help finding the plating solution that’s right for you, fill out this easy-to-use contact form.